- The decision was made after many child deaths in the Gambia and Uzbekistan.
The WHO warned that four Indian-made cough syrups were linked to the deaths. - The government has increased drug scrutiny and mandated testing on cough syrups before exporting them.
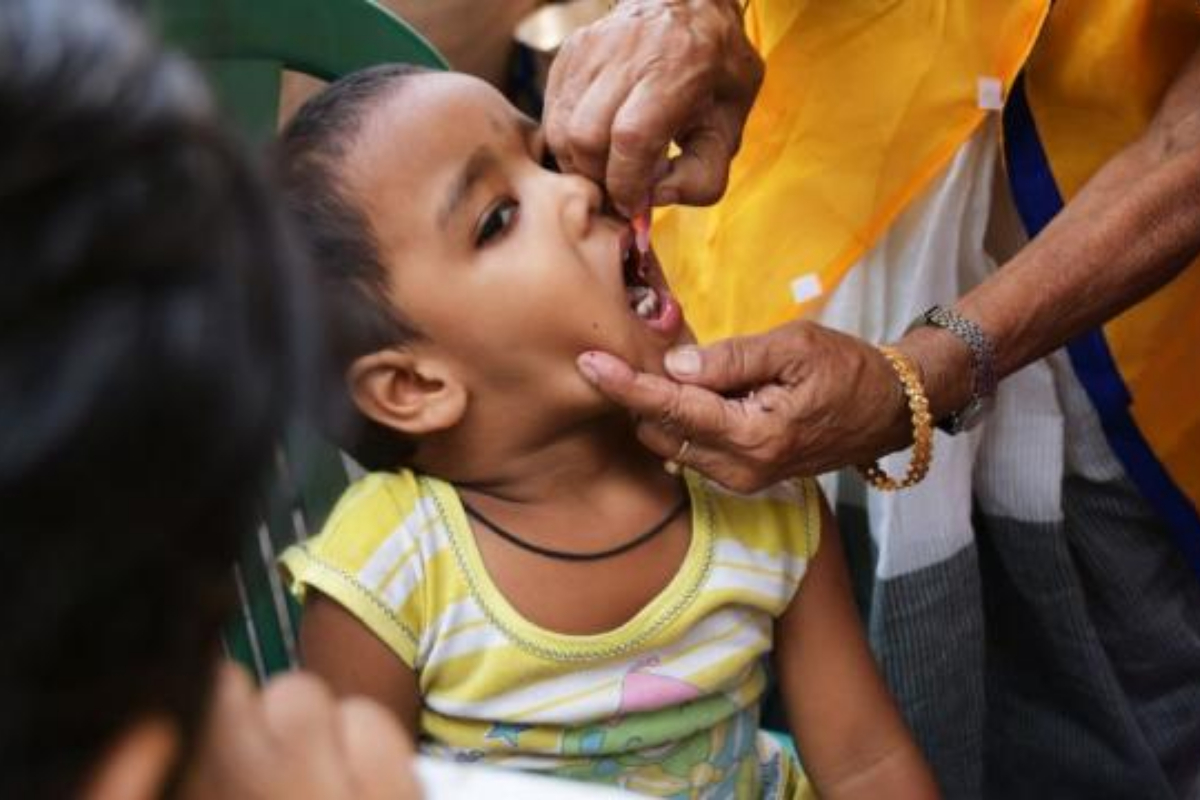
The highest drug regulatory authority in India’s highest drug regulatory authority has banned using anti-drugs for treating colds in children under four. This decision was made after many child deaths in the Gambia and Uzbekistan that were reportedly linked to cough syrups manufactured in India last year. In India, there were reports of at least 12 children who died between 2019 and 2020, allegedly after taking a similar medication.
Despite these concerns, the companies producing these drugs have refuted any allegations of wrongdoing and insist that their products are safe.
Fury in The Gambia over India cough syrup deaths:
A specific combination of three drugs—chlorpheniramine maleate and phenylephrine—that was approved in 2015 has now been banned. This combination is commonly used in cough syrups and tablets to address symptoms associated with the common cold.
As of Wednesday, a new directive has been issued, requiring pharmaceutical companies that produce and sell products containing this combination to include a warning label. This label must clearly state that the medication is not suitable for children under the age of four.
Why drugs made in India are sparking safety concerns:
Last year, there was increased scrutiny on cough syrups manufactured in India, prompted by a global warning from the World Health Organization (WHO). Four Indian-made cough syrups were reportedly linked to the tragic deaths of 66 children in The Gambia.
Laboratory analysis of syrup samples confirmed the presence of “unacceptable amounts” of diethylene glycol and another toxic alcohol called ethylene glycol.
Similar incidents occurred in Uzbekistan, where the health ministry reported 18 child deaths by 2022, allegedly linked to the consumption of an Indian-manufactured cough syrup.
In India’s Jammu region in 2019, at least 12 children, ranging from two months to six years old, lost their lives after reportedly ingesting a cough syrup believed to be toxic.
Long wait for justice after India cough syrup deaths:
Indian regulatory authorities have previously asserted that the reported deaths in the country were isolated incidents and not indicative of a broader issue.
Despite claims that the four cough syrups linked to child deaths in The Gambia met domestic specifications during testing, the World Health Organization (WHO) has challenged this assertion.
Nevertheless, the Indian authorities took action by revoking the manufacturing license of the company whose products were allegedly connected to fatalities in Uzbekistan.
To enhance oversight, the country has increased its scrutiny of drugs. In June, the government implemented a requirement for companies to conduct testing on their cough syrups before exporting them to other parts of the world.
[embedpost slug=”india-pm-narendra-modi-respond-to-murder-allegation-of-us-plot/”]