- An experimental medicine has delayed the rate of decline in memory and cognition among persons with early Alzheimer’s disease.
- It’s the first time medicine has been shown to influence the disease’s course.
- Eisai is recruiting people with a high risk of Alzheimer’s but no symptoms to take part in more trials.
In what is being characterized as a “historic moment” for dementia treatment, an experimental medicine has delayed the rate of decline in memory and cognition among persons with early Alzheimer’s disease.
The Eisai and Biogen medication reduced cognitive deterioration in Alzheimer’s patients by 27% after 18 months. It’s the first time medicine has been shown to influence the disease’s course.
“This is a historic moment for dementia research, as this is the first phase 3 trial of an Alzheimer’s drug in a generation to successfully slow cognitive decline,” said Dr. Susan Kohlhaas, the director of research at Alzheimer’s Research UK. “Many people feel Alzheimer’s is an inevitable part of aging. This spells it out: if you intervene early you can make an impact on how people progress.”
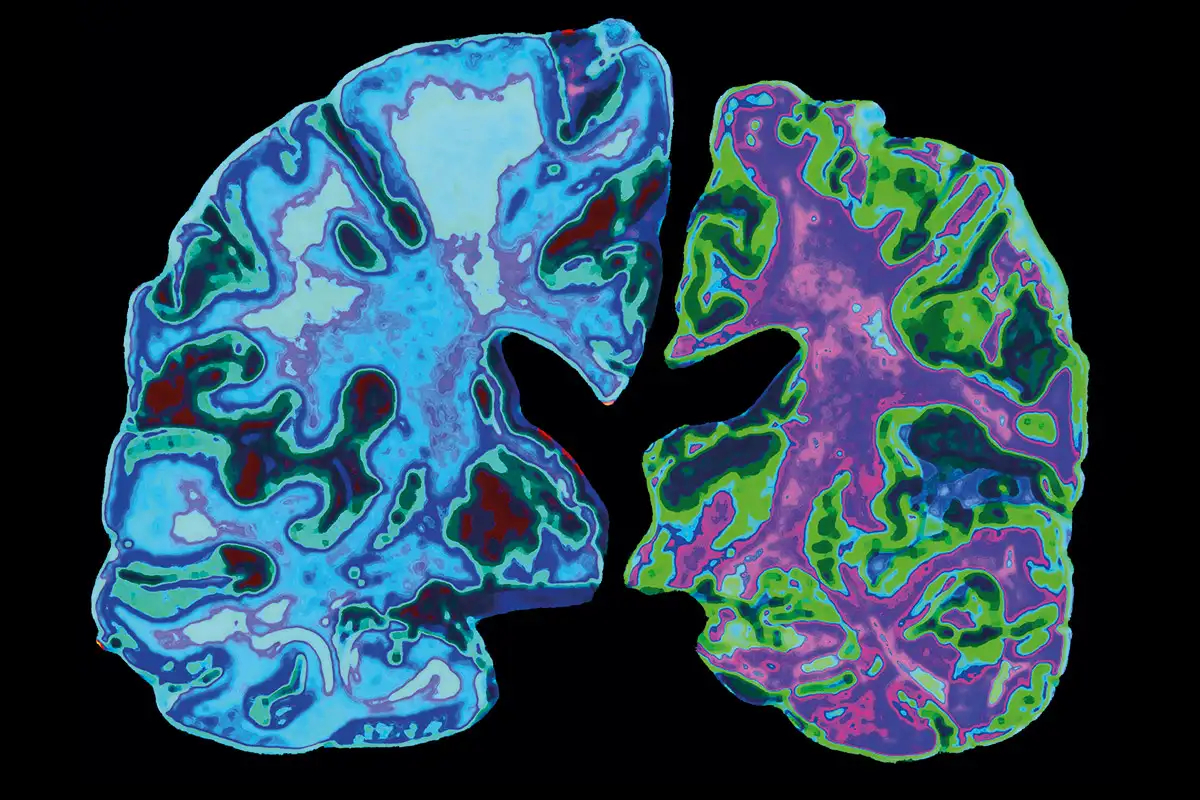
Infusions of lecanemab were administered on a twice-weekly basis to around 1,800 patients with early-stage Alzheimer’s disease in the research. It has also been found to lessen harmful plaques in the brain, halt patients’ memory loss, and improve their ability to do daily tasks.
About a fifth of individuals reported side effects, including brain enlargement or hemorrhage apparent on PET scans, with 3% reporting symptomatic side effects.
The results support the “amyloid hypothesis,” which says sticky plaques in dementia sufferers’ brains damage brain cells and cause cognitive loss.
Previous medication candidates reduced amyloid levels in the brain, but without improving clinical results, leading some to ask whether the research area was on the wrong route.
Rob Howard, a professor of geriatric psychiatry at University College London (UCL), stated, “This is an unambiguously statistically positive result and represents something of a historic moment when we see the first convincing modification of Alzheimer’s disease. God knows, we’ve waited long enough for this.”
By the end of the year, Eisai and Biogen are anticipated to submit regulatory approval applications in the United States and Europe. If approved, healthcare providers will have to decide whether to fund the medicine, which requires biweekly infusions, and who will be eligible for it because clinical improvements fall slightly below a commonly acknowledged criterion.
On a 14-point scale used to gauge Alzheimer’s progression, medication patients scored 0.45 higher than placebo patients, with an Alzheimer’s patient expected to drop 1 point a year.
Howard stated, “The accepted minimum worthwhile difference ranges from 0.5 to 1.0 points, [meaning] that there are going to be some very difficult conversations and decisions in the next weeks and months.”
Overall advantages will rely on whether patients continue to improve beyond 18 months, but the newest research can’t address that question.
There are also concerns over whether the medicine could reduce deterioration at an earlier stage. Eisai is recruiting people with a high risk of Alzheimer’s but no symptoms to take part in more trials.
The prospect of an effective Alzheimer’s therapy will focus attention on healthcare facilities’ ability to treat the 1 million persons affected in the UK — 1 in 14 adults 65 and older.
Only one in three psychiatry services would be ready to administer a new treatment within a year, and in the UK, many patients are diagnosed considerably later than those in the recent experiment.
Prof. Jon Schott, the chief medical officer of Alzheimer’s Research UK and a professor of neurology at UCL, said, “This will require a radical change in how we deliver our services.”
“If this is licensed and this gets through Nice [the National Institute for Health and Care Excellence], the demand will be huge. We’re not ready to deliver this at scale and we need to address that now.”
[embedpost slug = “/anabolic-steroids-ergogenic-drugs-and-protein-shakes/”]