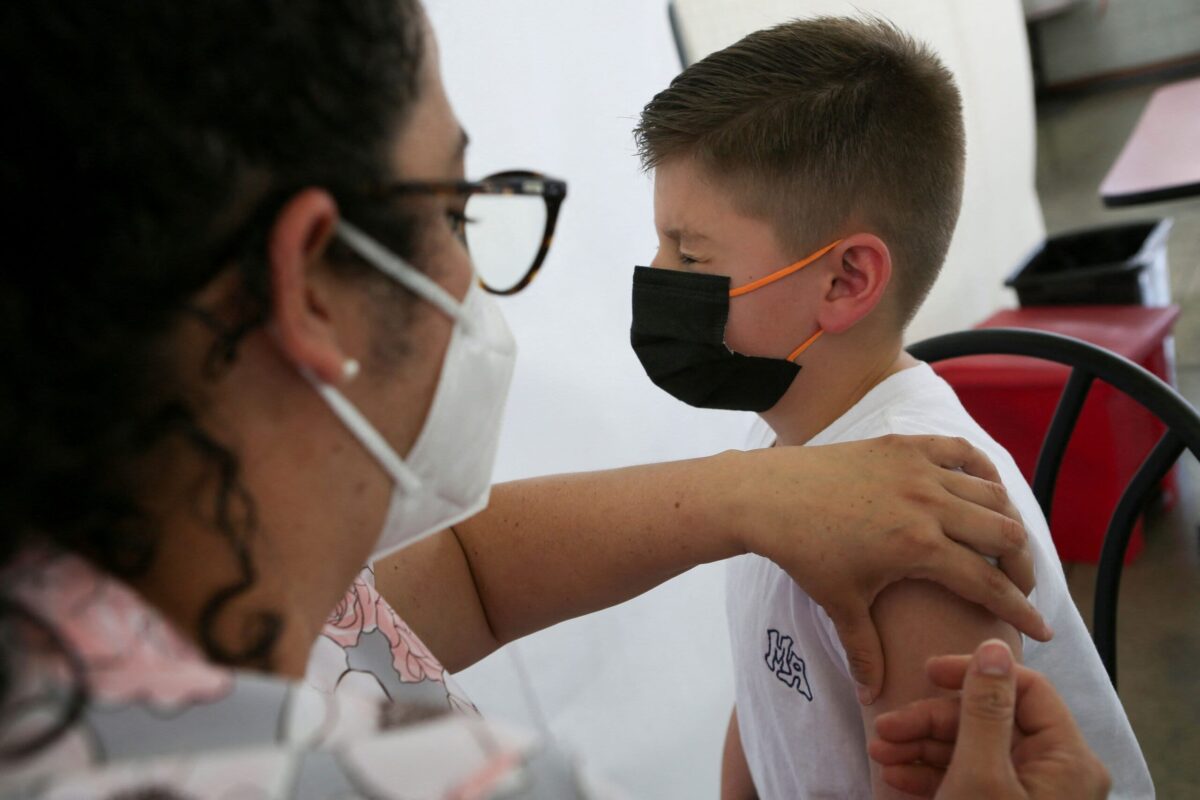
- The Pfizer and Moderna Covid-19 vaccines were cleared for use in children; aged five and younger.
- President Joe Biden greeted the move as a “monumental step” in the fight against Ebola.
- The vaccines required further clearance from the US Centers for Disease Control and Prevention.
The United States became the first country to approve the use of mRNA vaccines; for infants as young as six months old.
Pfizer and Moderna Covid-19 vaccines were cleared for children aged five and younger by US health officials; a move President Joe Biden hailed as a “monumental step” in the fight against the virus.
As a result, the United States became the first country to approve the use of mRNA vaccines for infants; as young as six months old.
The US Food and Drug Administration (FDA) approved their emergency use for young children.
Read More: India approves Corbevax as a Covid booster
The vaccines, however, required additional approval from the US CDC, which was granted.
“We know that millions of parents and caregivers; who want to get their young children vaccinated; and with today’s decision; they will be able to do so,” CDC Director Rochelle Walensky said.
Read More: Germany rolls out COVID-19 vaccines for children